Tyvek® Outperforms Medical-Grade Paper in Test After Test
Article | February 16, 2018
Compared to medical-grade papers, DuPont™ Tyvek® offers an optimum balance of microbial penetration resistance, tear strength, puncture resistance and clean peel. And unlike medical-grade papers and films, Tyvek® is compatible with all of the most commonly used sterilization methods, including: ethylene oxide (EO), gamma, electron-beam, steam (under controlled conditions) and low-temperature oxidative sterilization processes.
Tyvek® consistently outperforms medical-grade papers in tests for microbial barrier, tear and puncture resistance, moisture resistance and particle generation.
The secret to the superior performance of Tyvek® is that it’s not a paper, but rather a sheet of flashspun and bonded high-density polyethylene (HDPE) filaments. Continuous strands of very fine, interconnected filaments are randomly oriented and bonded together by heat and pressure during manufacture. The result is a tough, durable sheet structure that provides a unique combination of physical properties that no other sterile packaging material can match.
Excellent Barrier to Microbial Penetration
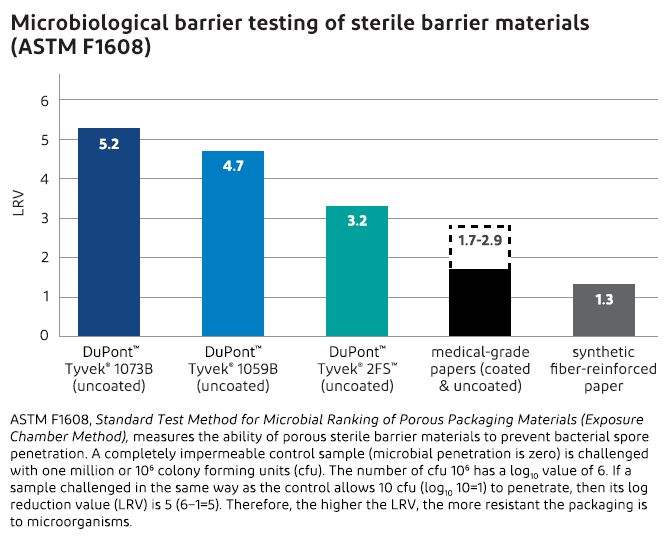
The number-one priority in selecting packaging materials for medical devices is the ability of the package to maintain sterility from the point of sterilization until the product is opened for use. Even under the most rigorous conditions in highly contaminated environments, Tyvek® is highly resistant to penetration by bacterial spores and other contaminating microorganisms.
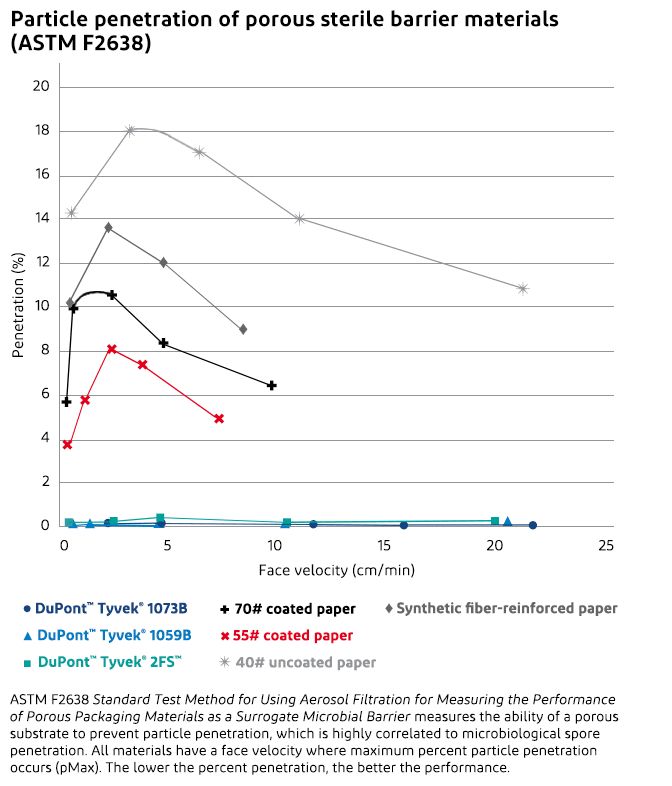
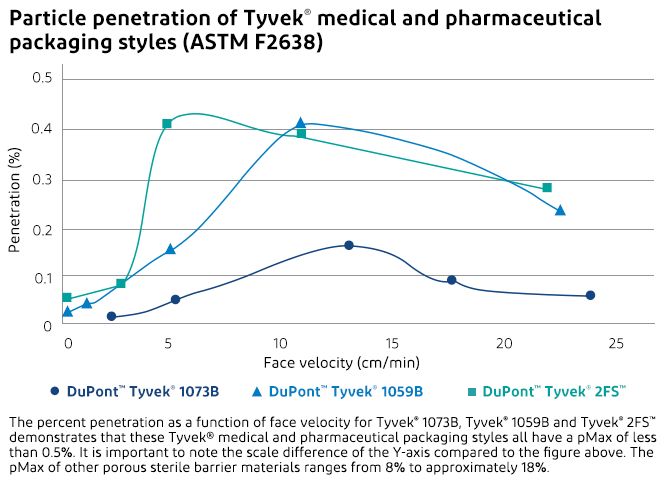
Particulate and bacteriological tests clearly demonstrate that Tyvek® outperforms other commercially available porous packaging materials, including medical-grade papers.
Comprehensive shelf-life studies have shown that Tyvek® can maintain sterility for at least five years if package integrity is not compromised.
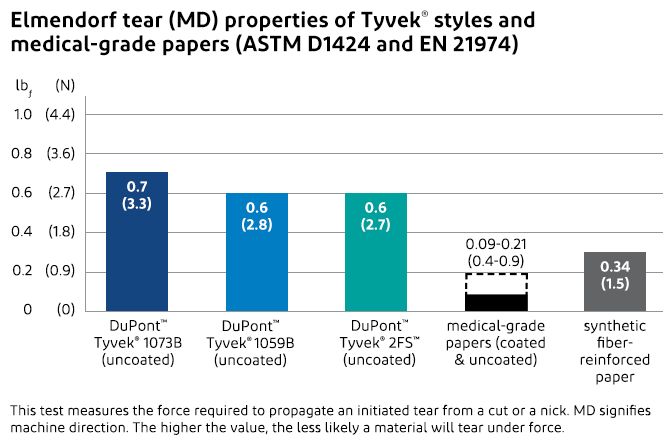
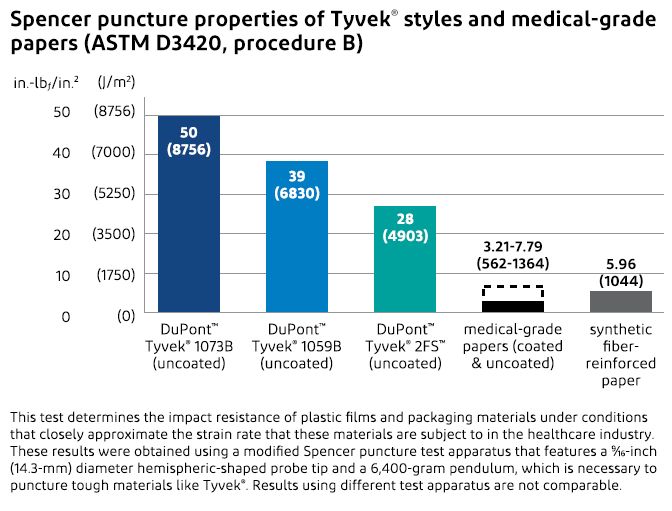
Superior Tear Strength and Puncture Resistance
The tough, continuous filaments of Tyvek® protect packages from product breakthrough and also from penetration by an object outside the packaging during rough handling. Compared to medical-grade papers, Tyvek® has superior puncture resistance and tear strength, which means that Tyvek® does not puncture easily and tears do not readily propagate if a package is nicked.
Exceptional Resistance to Breakage
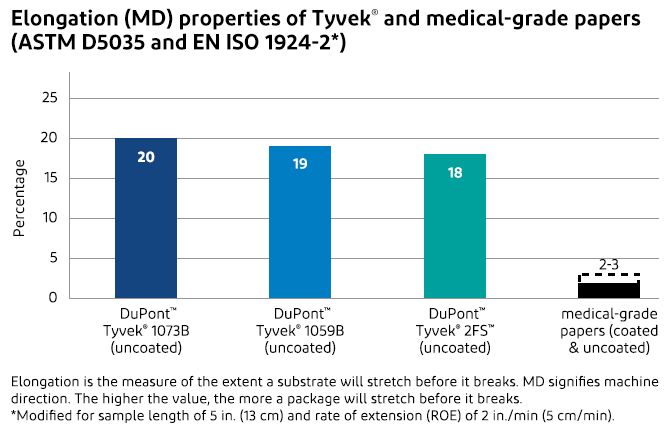
Tyvek® is an extremely flexible packaging material that won’t break or tear as easily as medical-grade papers. This resiliency, combined with the inherent strength of Tyvek®, ensures that form-fill-seal packaging lines run smoothly and without significant downtime because of material web breaks.
Outstanding Moisture Resistance and Moisture Vapor Transmission
Tyvek® is highly resistant to penetration by water. In fact, water in contact with Tyvek® does not wet its surface or spread; it simply remains as droplets on the surface. That’s because Tyvek® is hydrophobic and does not absorb moisture, giving it distinct advantages compared to medical-grade paper. For example, when medical-grade paper absorbs moisture, its strength and puncture resistance are reduced. This can greatly influence package performance, especially during distribution. In sharp contrast to medical-grade papers, Tyvek® maintains its superior strength both wet and dry.
Another advantage of Tyvek® vs. medical-grade paper is that a high moisture vapor transmission rate (MVTR) can be achieved with Tyvek®. This is particularly important for the ethylene oxide (EO) sterilization process. In the EO process, water is introduced as a vapor because moisture enhances the effectiveness of EO as a sterilant. Because medical-grade paper absorbs moisture vapor, it does not allow for a high MVTR across the material to the package contents.
Low-temperature oxidative sterilization processes such as hydrogen peroxide gas plasma cannot be used with cellulosic materials such as medical-grade paper because they rapidly interact with the oxidizing agent and its superoxide radicals. This can result in an aborted cycle because of inadequate sterilant concentration. Tyvek® is high-density polyethylene (HDPE) and allows for an effective sterilant concentration to be achieved. Although Tyvek® interacts only slightly with these types of sterilants, the surface energy is increased. This can cause a lowering of the hydrostatic head in industrial applications where multiple sterilization cycles are validated. The performance of Tyvek® as a sterile barrier is not affected but the liquid test methods for assessing package integrity (such as dye penetration and water immersion) can produce results that are different from untreated material.
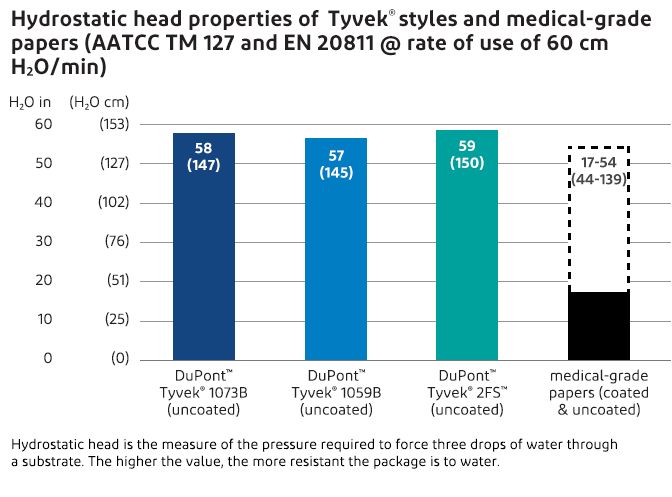
Tyvek® has superior hydrostatic head properties compared to medical-grade papers. Hydrostatic head is the measure of the pressure required to force three drops of water through a substrate. The higher the value, the more resistant the package is to water.
Clean Peel
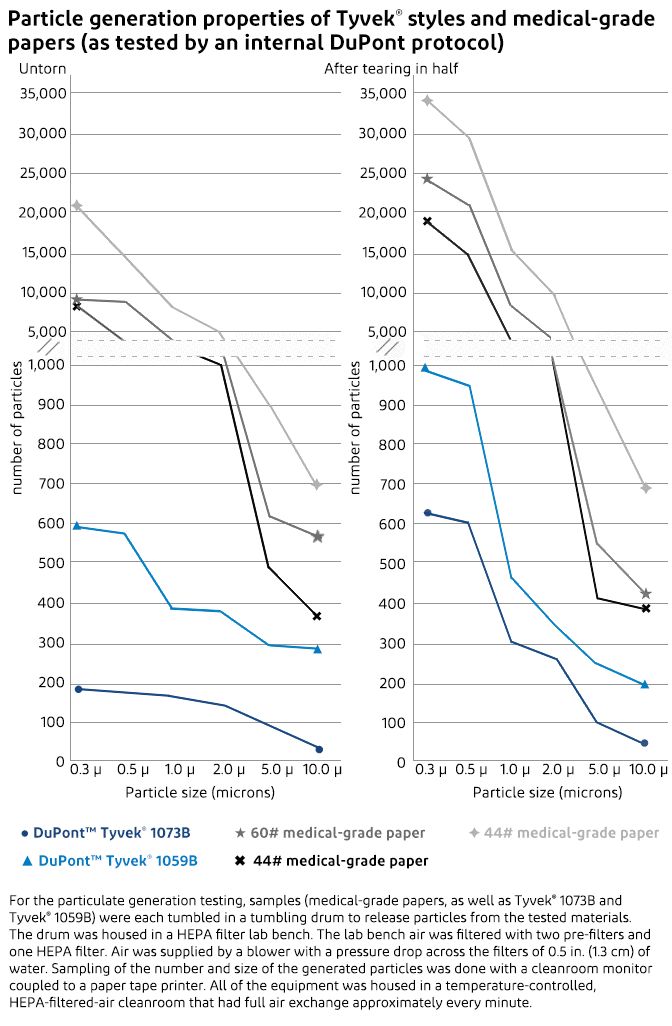
Unlike medical-grade paper, which can release a significant number of particulates when a package is opened, the unique, continuous filament structure of Tyvek® results in clean peel and low lint features that minimize the risk of device contamination when packages are opened or handled.
Particulate generation tests comparing Tyvek® to medical-grade papers showed conclusively that Tyvek® generates far fewer airborne particulates that could contaminate the medical device or the sterile field. The particulate generation testing measured the quantity and the size of particles generated by Tyvek® and medical-grade papers both before and after being torn in half. The tests proved that Tyvek®—with its clean peel and low-linting properties—generates significantly fewer airborne particulates than medical-grade papers.