Practice makes perfect, but is it enough?
By Jeff Humphries
Distribution Safety and Emergency Response Specialist, Veolia North America

Most professional sports teams start the season with weeks of rigorous practice. They spend hours upon hours in a classroom setting, reviewing every game-day scenario imaginable. But even with all this groundwork, nothing prepares a team for regular-season play like a pre-season scrimmage. A scrimmage generates real-life situations and provokes important questions. Was the game plan right? Were there logistical or equipment issues? How were timing and communication?
The same approach can be applied by hazmat teams and emergency-response organizations at the public, chemical, manufacturing and consumer levels. All emergency-response organizations practice. We practice by attending tabletop and classroom discussions. We practice by simulating incidents. We practice by plugging and patching water leaks. We practice by donning personal protective equipment (PPE). We're creative with our drills to help us prepare for "game day." Many of us are required to do so by law as part of emergency response planning and 29 CFR 1910.120.
We can simulate hazmat responses, but does it prepare us for reality? We wonder if it's possible to get hands-on training in near-life scenarios. We ask how it's possible to practice mitigation techniques on hazardous materials if we don't have a live event. Well, it's possible through a scrimmage.
Veolia North America's Acid Technology Center will host a scrimmage June 2–16, 2019. The 2019 Fuming Acids Spill Mitigation Workshop, also known as Spill School, offers attendees the chance to mitigate, neutralize and clean up live releases of fuming hazardous materials, including fuming sulfuric acid (65% oleum) and chlorosulfonic acid. Mitigation training for these live releases features fume knockdown and suppression; application of water mist and/or foam; addition of neutralization agents; and complete cleanup.
Held at the Department of Energy's Nonproliferation Test and Evaluation Complex facilities in Mercury, Nevada, this workshop gives attendees the opportunity to safely experience a live release of toxic inhalation hazard chemicals outside of an actual emergency. Spill School is open to emergency responders, chemical manufacturers, fuming acid end users, transportation companies and hazmat customers.
Although a reduction in incidents is evidence of a strong safety culture, it also highlights the need for practice. Don't get caught on your heels. If you believe practice makes perfect, consider adding a scrimmage to your hazmat-response training routine. The hands-on opportunity provided by Veolia has proven to be valuable for many in gaining necessary game-day experience.
Don't delay! Spill School registration ends on April 5, 2019. For more information, please contact jeffrey.humphries@veolia.com.
Taping problems? Glove rings might be the answer
By Daniel Bowen
Technical Specialist, DuPont Personal Protection
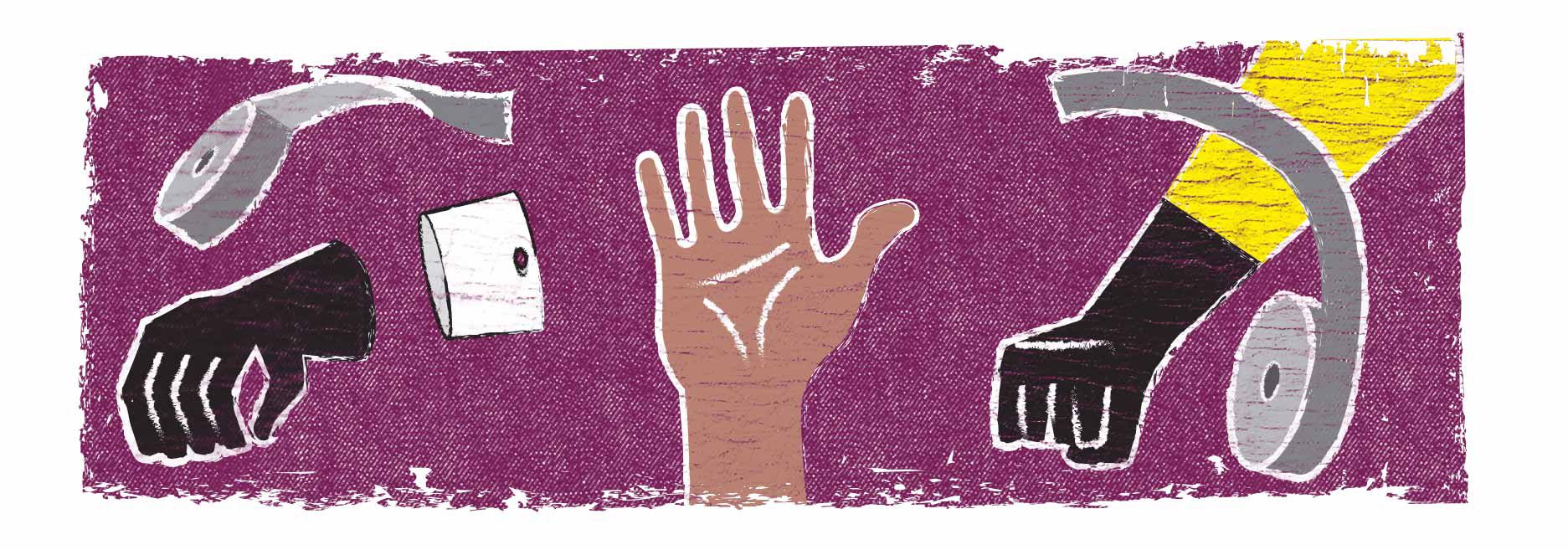
Taping chemical protective suit sleeves and gloves together may present some challenges. Many protective apparel users will put a glove on their hand, pull on their suit, and then ask an assistant to gather up any extra fabric and apply tape. This situation can result in three different problems:
- The suit's fabric bunches and wrinkles at the wrist. Wrinkles are a potential point of entry or leak source.
- The wearer's range of motion is restricted. If the wearer reaches for something, the tape may pop loose, creating another potential leak source.
- If a medical emergency occurs and the wearer needs to get out of their suit quickly, they may encounter difficulty doing so, further risking their safety.
That's where glove rings come in. To use a glove ring, the wearer simply slips a glove onto the ring and tapes it in place using chemical protective tape or duct tape. The wearer then slips the ring and glove combination over their hand. When they pull the chemical protective suit over this ensemble, the sleeve fabric will stop halfway up the ring. Finally, the wearer puts a layer of tape over the ring and suit union and they've addressed all three problems outlined above.
The wrinkles are gone and the wearer now has full range of motion. If they need to get out of their suit quickly, they can easily take their hand out for a quick exit.
This short video provides a brief demonstration on how to use the glove rings and how glove rings may address some of these common taping problems.
Hazmat responders and flash fire protective clothing—what do flame resistance claims really mean?
By Khyati Vyas
Research Investigator, DuPont Personal Protection

We live in a world where many hazards are not just one-dimensional. In the hazmat response world, dual chemical and flame hazards are often quite common, whether in the oil & gas/petrochemical industry, law enforcement, meth labs or transportation of shipments. And, they come in a variety of forms.
Hazmat response scenarios often involve materials that are considered flammable. Consider enclosed spaces where the concentration is above the lower flammable limit (LFL). Picking chemicals that clearly have dual chemical and flame threats is easy. If you use the National Fire Protection Association (NFPA) diamond for information, anything with 3 or 4 in the flammability diamond would be a concern. However, the flammable and toxic/corrosive hazards don't have to be in the same molecule, just in the same vicinity. If there is a chemical hazard in the vicinity of a flame hazard, then you have a dual chemical and flame hazard scenario. That's why it's critical to choose the appropriate chemical resistant (CR) and flame resistant (FR) personal protective equipment (PPE) based on the hazards present.
Why even consider the FR characteristics of your hazmat suit? Most materials used in traditional CR suits are flammable and meltable. Most materials used in traditional FR clothing offer little or no chemical protection. If heat/flame and chemical hazards are present, never wear a traditional CR suit over or under a traditional FR suit. If the hazard risk assessment shows that chemical barrier protection is needed (based on permeation data) and flame resistance is needed, then special dual CR/FR PPE protection will be required.
Safety is the number one priority; that is why when we discuss FR claims, it is critical that the hazard's FR needs match the suit's FR performance level. This can mean a variety of different things, and all go back to the hazard risk assessment and the identified requirements. Does the suit need to:
- Not ignite when exposed to a flame?
- May ignite when exposed to a flame, but will quickly self-extinguish with minimal damage once the flame source is removed?
- Not melt and drip?
- Remain intact and provide thermal insulation to protect the wearer's skin to reduce the likelihood of thermal burn injury?
- Remain intact and continue to hold out remaining chemical hazards (vapor or liquid)?
If you have a dual chemical and flame hazard, your risk assessment should consider the likelihood of chemicals igniting (or reacting) when personnel are present. Several NFPA standards for PPE can assess response to flame exposure to predict the chemical protective clothing's ability to aid the wearer to escape with minimal harm. Hazard assessments should verify that both the "CR" properties and "FR" properties of the chosen PPE fully match the identified hazard(s).
Finally, make sure the suit manufacturer fully states the level of CR and the level of FR performance to expect. Checking and understanding the performance data is key for protection.
In future articles, we will explore the NFPA standards to assess the FR properties of chemical protective clothing and the different types of FR test methods used by the NFPA for chemical protective clothing.
Hazmat through history: Meth labs
By Daniel Hammel
Marketing Consultant, DuPont Personal Protection
Developed in Japan in 1893, methamphetamine, commonly known as meth, was used medically for asthma, narcolepsy and weight loss. It was also used by several countries during World War II to help keep soldiers awake. For example, Japanese pilots would take high doses of the drug before their kamikaze suicide missions.
Through the 1950s and 1960s, methamphetamine continued to be used medically as a diet aid and for depression. Nonmedically, it remained in use as a stimulant to help students, athletes and truck drivers stay awake. However, due to widespread abuse and addiction, the U.S. made it illegal in the 1970s.
Drug trafficking organizations eventually set up meth labs to produce large quantities of this highly addictive drug. California was a hotbed for these labs, eventually becoming home to 97 percent of the drug's "super labs." More recently, the substance has increasingly been manufactured in "home labs" to produce small batches. These smaller scale meth labs can be found in residential neighborhoods, parks, forests and even automobiles.
In 2012, police in Lakeland, Florida, initially thought that a car crash had killed the driver of a car. Upon closer inspection, evidence of a "shake and bake" meth kit was discovered inside the vehicle. Investigators found that an explosion from the mixing and shaking of chemicals in a bottle caused shards of glass to kill the driver, which in turn caused the car to crash.
Regardless of the size, meth labs are dangerous for the people around them. Illicit drug manufacturers are not the only people in harm's way; nearby neighbors, law enforcement officials and hazmat response workers are also at risk of serious or fatal injury. Labs are highly flammable and also have dermal and respiratory chemical hazards. It can take weeks for a hazmat crew to clean up the byproducts of a single lab, and the area around a lab can remain toxic for years after its last production.
To find out which DuPont products offer suitable protection for hazardous chemicals and/or CR/FR threats, please visit safespec.dupont.com.
Products and/or sales questions?
Share your stories, tips and tricks.

Resource library
Find technical information, videos, webinars and case studies about DuPont PPE here.


